进口保健食品在境外转让流程图
时间:2022-02-19 浏览:1,688
导读:
进口保健食品在境外转让流程图
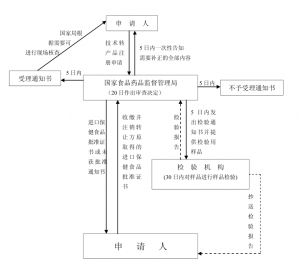
进口保健食品在境外转让流程图
责任编辑:本站小编
相关文章:
- [流程周期]保健食品原注册人产品转备案流程
- [流程周期]进口保健食品备案申请条件及申报流程
- [流程周期]保健食品注册申请条件及申报流程
- [流程周期]保健食品注册的法律界定、申报流程及资料要求
- [流程周期]保健食品备案信息填报流程图及备案资料形式要求
- [流程周期]天健华成小课堂:保健食品备案流程一览
- [流程周期]对改变产品名称、保质期、食用量,缩小适宜人群范围,扩大不适宜人群范围、注意事项以及功能项目的变更申请(进口)流程
- [流程周期]对改变产品规格、质量标准以及进口保健食品生产厂商在中国境外改变生产场地的变更申请(进口)
- [流程周期]进口保健食品在境内转让流程图
- [流程周期]保健食品审批流程图
相关推荐:
- [常见问题]保健食品含片与颗粒剂溶化性指标检测、标志性成分或功效成分检测方法学研究资料、按新版食品安全国家标准修改指标的产品,应提供哪些申报资料?
- [常见问题]关于化妆品申报资料、用户名密码补发有关问题
- [常见问题]若化妆品标签存在《办法》第二十条规定的瑕疵,企业可以采取哪些改正措施?
- [流程周期]对改变产品名称、保质期、食用量,缩小适宜人群范围,扩大不适宜人群范围、注意事项以及功能项目的变更申请(进口)流程
- [申报资讯]国家食药局”三定”尘埃落定,升格正部级,进口非特殊化妆品审批权下放省局
- [申报资讯]国家药品监督管理局行政受理服务大厅关于2025年端午节放假安排的公告(第356号)
- [常见问题]保健食品检验报告内容更正有何要求?
- [常见问题]保健食品试验检验脏器称量指标中为何不包括雌性动物卵巢重量?
- [审批动态]2024年8月13日保健食品注册批件(决定书)待领取信息
- [申报资讯]保健食品审评又换新地址
保健食品申报频道
联系我们
-
86-010-84828041/42
400-6167-168
zhuceabc@zhuceabc.com
咨询微信:
13601366497(化妆品类)
1801335159(特殊食品类)
最新更新
热门排行











