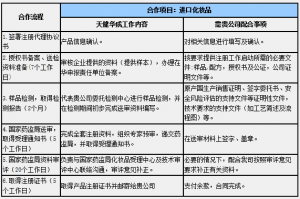
业务合作流程(以进口化妆品申报为例)
时间:2022-02-21 浏览:1,732
导读:
责任编辑:本站小编
相关文章:
相关推荐:
- [政策法规]《保健食品命名指南(2019年版)》(全文)
- [申报资讯]国家药品监督管理局行政受理服务大厅2023年端午节放假安排的公告(第328号)
- [政策法规]关于进一步明确保健食品有关辅料替代工作要求的通知
- [申报资讯]化妆品标签违法违规典型案例分析
- [申报攻略]国产保健食品批文申报注册10步走(天健华成)
- [流程周期]我国化妆品备案注册申报背景说明
- [常见问题]进口保健食品由境外厂商常驻中国代理机构办理注册事务或委托境内代理机构负责办理注册事项的,应加盖谁的印章?
- [News]SAMR Issued the Provisions for Registration and Notification of Cosmetics
- [审批动态]2022年03月07日化妆品批准证明文件邮寄详情单
- [政策法规]保健食品备案剂型粉剂的技术要求(2020年版)(征求意见稿)及其起草说明
保健食品申报频道
联系我们
-
86-010-84828041/42
400-6167-168
zhuceabc@zhuceabc.com
咨询微信:
13601366497(化妆品类)
1801335159(特殊食品类)
最新更新
热门排行











