原卫生部《进口非特殊用途化妆品备案凭证》
时间:2022-02-22 浏览:1,465
导读:
原卫生部《进口非特殊用途化妆品备案凭证》
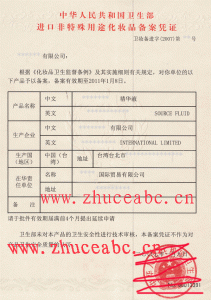
原卫生部《进口非特殊用途化妆品备案凭证》
责任编辑:本站小编
上一篇:« Main Responsibilities of the National Medical Products Administration
下一篇:2011CFDA《进口非特殊用途化妆品备案凭证》 »
下一篇:2011CFDA《进口非特殊用途化妆品备案凭证》 »
相关文章:
- [化妆品]2022国家药品监督管理局特殊化妆品注册证样式(天健华成提供)
- [化妆品]2019《进口非特殊用途化妆品备案凭证》式样
- [化妆品]2018版进口非特殊用途化妆品备案凭证
- [化妆品]国产特殊用途化妆品行政许可批件样本
- [化妆品]自贸区进口非特殊用途化妆品备案电子凭证样本
- [化妆品]国产非特殊化妆品备案凭证样式(纸质)
- [化妆品]原CFDA《特殊用途化妆品行政许可批件》样式
- [化妆品]2011CFDA《进口非特殊用途化妆品备案凭证》
- [化妆品]自贸区进口非特殊用途化妆品备案电子凭证样本
相关推荐:
- [审批动态]2024年06月19日化妆品注册批准证明文件(变更、纠错)送达信息
- [审批动态]2022年03月18日化妆品批准证明文件待领取信息发布
- [申报资讯]保健食品注册申报代理
- [常见问题]申报终产品为阿胶的保健食品,需注意什么?
- [申报资讯]国家药监局|《牙膏监督管理办法》解读(二)
- [费用预算]进口普通化妆品备案需要多少费用?
- [审批动态]2023年04月14日化妆品批准证明文件送达信息发布
- [申报资讯]市场监管总局发布《动物源性食品中瓜尔胶的测定》等10项食品补充检验方法公告
- [申报资讯]儿童化妆品标志AI设计图可以下载了!
- [申报攻略]天津市进口非特殊用途化妆品备案服务指南
化妆品申报频道
联系我们
-
86-010-84828041/42
400-6167-168
zhuceabc@zhuceabc.com
咨询微信:
13601366497(化妆品类)
1801335159(特殊食品类)
最新更新
热门排行











